Dr. Stuart Titus, Ph.D., is an industry innovator who has been involved in the growth of many companies, including Kannaway.
Previously a Wall Street veteran, where he was a bond trader for 11 years, Dr. Titus’ diverse background in both finance and healthcare is the impetus behind his strong passion to help others by supporting the greater cannabis industry.
Dr. Titus is an expert in cannabinoids, and the endocannabinoid system, and is regularly asked to keynote speak at major medical conferences around the world. With a fellowship with the American Academy of Pain Management and clinical association with the American Association of Integrative Medicine, Dr. Titus also previously practiced as a Physiotherapist. For over fifteen years, he ran clinics that specialised in integrative pain management and injury rehabilitation, treating over 40,000 patients. His first-hand experience with therapeutic hemp oil products as nutritional supplements led him to be a forerunner in the industrial hemp industry in the United States and around the world.
HEMP IN EVERY HOME
For most of the 20th century, cannabis plants, such as industrial hemp, were widely misunderstood, which lead to many people not seeing the incredible potential it holds. With thousands of practical uses, including wellness, nutrition, fibres, textiles, and environmentally friendly construction materials, Kannaway’s goal is to help make hemp an essential part of commerce and agriculture once again. With this goal in mind and driven by a small group of hemp pioneers, Kannaway began our journey to bring cannabis solutions back to the world again and to every home.
WHAT IS THE NOVEL FOOD LEGISLATION?
A regulation first introduced in 1997, later updated on 1 January 2018 with the aim of establishing a food safety mechanism to control newly developed, synthetic, or genetically produced food, and it applies to all food that has not been used to a significant degree before 15 May 1997 for human consumption.
Any food that is considered novel must be authorised by the EU commission and undergo a pre-market safety assessment by the European hood Safety Authority ESA before it can be legally marketed in the EU. The Novel Food Catalogue serves as a non-legally binding orientation on whether a product (of animal and plant origin, as well as other substances) will need an authorisation under the Novel Food Regulation. The Novel Food Catalogue reflects the opinion of the Member States
IS CBD A NOVEL FOOD?
Until the end of 2018 extracts of cannabidiol were considered novel ONLY if the levels of cannabidiol were higher than the BD levels in the source Cannabis sativa L.
The Standing Committee already decided in December 1997 and the Commission confirmed to the European hemp industry in writing in the beginning of 1998 it was decided that foods containing parts of the hemp plant do not fall under the scope of the regulations EC 258/97″ and also “that hemp flowers… are considered to be food ingredients”. Hemp flowers and leaves, as part of the hemp plant, were not considered to be Novel Food.
Cannabinoid extracts with naturally occurring level of cannabinoids and traditionally produced hem extracts were already on the market and consumed before 99/ to a significant degree.
In January 2019, Member States representatives updated the Catalogue entries for Cannabis sativa L.” and “Cannabinoids” incorrectly and the European Industrial Hemp Association (EIHA) have repeatedly explained to Member States and the European Commission based on logic and historical facts. why this is incorrect.
THE EIHA NOVEL FOOD CONSORTIUM
Because of this situation, a legal and planning security for the European hemp industry and for the trade of CBD-related-products can only be achieved by approval as Novel Food. As such, EIHA have set up a consortium which Kannaway is a contributing member, and have submitted a joint Novel Food application. Find out more here: https://eiha.org/status-ot-hemp-
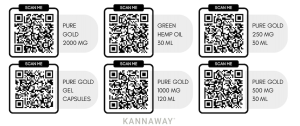
The following products have already been reviewed and approved to be part of a submitted, ongoing Novel Foods application:
Pure Gold 2000 MG
Green Hemp Oil 30ML
Pure Gold 250MG 30ML
Pure Gold Gel Capsules
Pure Gold 1000MG 120ML
Pure Gold 500MG 30ML